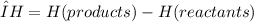Chemistry, 29.11.2019 00:31 foralways3348
An endothermic reaction has an endothermic reaction has a positive δh, absorbs heat from the surroundings, and feels cold to the touch. a positive δh, absorbs heat from the surroundings, and feels warm to the touch. a positive δh, gives off heat to the surroundings, and feels warm to the touch. a negative δh, absorbs heat from the surroundings, and feels cold to the touch. a negative δh, gives off heat to the surroundings, and feels warm to the touch.

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3
You know the right answer?
An endothermic reaction has an endothermic reaction has a positive δh, absorbs heat from the surroun...
Questions





Mathematics, 16.10.2019 22:30







History, 16.10.2019 22:30

Mathematics, 16.10.2019 22:30

Chemistry, 16.10.2019 22:30

Mathematics, 16.10.2019 22:30


Mathematics, 16.10.2019 22:30


Mathematics, 16.10.2019 22:30