
Chemistry, 29.11.2019 02:31 mathman783
Given that δ h ∘ f [ br ( g ) ] = 111.9 kj ⋅ mol − 1 δ h ∘ f [ c ( g ) ] = 716.7 kj ⋅ mol − 1 δ h ∘ f [ cbr 4 ( g ) ] = 29.4 kj ⋅ mol − 1 calculate the average molar bond enthalpy of the carbon‑bromine bond in a cbr 4 molecule.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Given that δ h ∘ f [ br ( g ) ] = 111.9 kj ⋅ mol − 1 δ h ∘ f [ c ( g ) ] = 716.7 kj ⋅ mol − 1 δ h ∘...
Questions

Health, 30.09.2019 15:30

Mathematics, 30.09.2019 15:30


Mathematics, 30.09.2019 15:50


Mathematics, 30.09.2019 15:50



Biology, 30.09.2019 15:50



Social Studies, 30.09.2019 15:50


Business, 30.09.2019 15:50


Mathematics, 30.09.2019 15:50


History, 30.09.2019 15:50


History, 30.09.2019 15:50

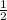 Br2(g) ⇒ Br(g) , Δ H ∘ = 111.9 kJ ⋅ mol − 1
Br2(g) ⇒ Br(g) , Δ H ∘ = 111.9 kJ ⋅ mol − 1 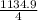 = 283.725 kJ ⋅ mol − 1
= 283.725 kJ ⋅ mol − 1

