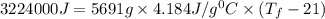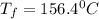The balanced combustion reaction for c 6 h 6 is 2 c 6 h 6 ( l ) 15 o 2 ( g ) ⟶ 12 co 2 ( g ) 6 h 2 o ( l ) 6542 kj if 7.700 g c 6 h 6 is burned and the heat produced from the burning is added to 5691 g of water at 21 ∘ c, what is the final temperature of the water

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1


Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
The balanced combustion reaction for c 6 h 6 is 2 c 6 h 6 ( l ) 15 o 2 ( g ) ⟶ 12 co 2 ( g ) 6 h 2 o...
Questions

Mathematics, 21.05.2021 20:40

Mathematics, 21.05.2021 20:40


Mathematics, 21.05.2021 20:40

Physics, 21.05.2021 20:40

Mathematics, 21.05.2021 20:40

Mathematics, 21.05.2021 20:40


Mathematics, 21.05.2021 20:40


English, 21.05.2021 20:40

Mathematics, 21.05.2021 20:40

Mathematics, 21.05.2021 20:40

Mathematics, 21.05.2021 20:40


Mathematics, 21.05.2021 20:40


Mathematics, 21.05.2021 20:40

Mathematics, 21.05.2021 20:40

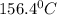
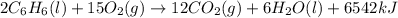
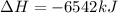
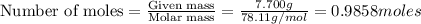
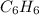 releases = 6542 kJ of heat
releases = 6542 kJ of heat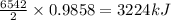 of heat
of heat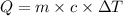
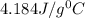
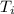 = 21.0°C
= 21.0°C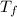 = ?
= ?