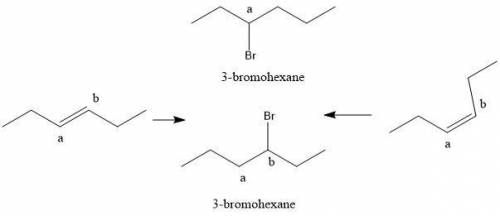
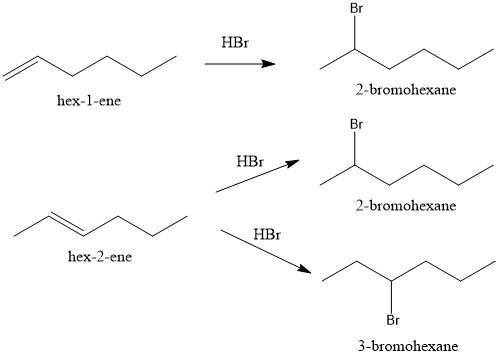
Compounds x and y are stereoisomers having the formula c6h12. both x and y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form hexane, and they each react with hbr to give a single bromoalkane product. draw structural formulas for both x and y.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3


Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
Compounds x and y are stereoisomers having the formula c6h12. both x and y react with one molar equi...
Questions



Spanish, 15.10.2020 03:01

English, 15.10.2020 03:01


Mathematics, 15.10.2020 03:01

Chemistry, 15.10.2020 03:01


Mathematics, 15.10.2020 03:01

Mathematics, 15.10.2020 03:01



English, 15.10.2020 03:01

Mathematics, 15.10.2020 03:01