
Aspirin (acetylsalicylic acid, c9h8o4) is a weak monoprotic acid. to determine its acid-dissociation constant, a student dissolved 2.00 g of aspirin in 0.600 l of water and measured the ph. what was the ka value calculated by the student if theph of the solution was 2.62? a 0.100 m solution of ethylamine (c2h5nh2) has a ph of 11.87. calculate the kb for ethylamine.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

You know the right answer?
Aspirin (acetylsalicylic acid, c9h8o4) is a weak monoprotic acid. to determine its acid-dissociation...
Questions






Social Studies, 29.06.2021 16:10

Physics, 29.06.2021 16:10

Mathematics, 29.06.2021 16:10



Computers and Technology, 29.06.2021 16:10


Chemistry, 29.06.2021 16:10

Business, 29.06.2021 16:10


Mathematics, 29.06.2021 16:10

Biology, 29.06.2021 16:10



English, 29.06.2021 16:10

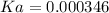
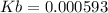
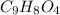 = 180.16 g/mol
= 180.16 g/mol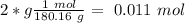
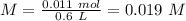
![Ka=\frac{[X][X]}{[0.019-X]}=\frac{[X]^2}{[0.019-X]}](/tpl/images/0395/6760/646b6.png)
![pH=-Log[H^+]](/tpl/images/0395/6760/3ca39.png)
![[H^+]=10^-^p^H](/tpl/images/0395/6760/16952.png)
![[H^+]=10^-^2^.^6^2=0.00240](/tpl/images/0395/6760/b62ea.png)
![Ka=\frac{[0.00240]^2}{[0.019-0.00240]}=0.000346](/tpl/images/0395/6760/6c4c9.png) .
.![Kb=\frac{[X][X]}{[0.1-X]}=\frac{[X]^2}{[0.1-X]}](/tpl/images/0395/6760/c1bde.png)
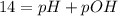
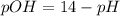
![pOH=-Log[OH^-]](/tpl/images/0395/6760/626fd.png)
![[OH^-]=10^-^p^O^H](/tpl/images/0395/6760/86a8e.png)
![[OH^-]=10^-^2^.^1^3=0.00741](/tpl/images/0395/6760/49ab9.png)
![Kb=\frac{[0.00741]^2}{[0.1-0.00741]}=0.000593](/tpl/images/0395/6760/bbeaa.png)


