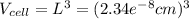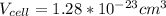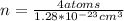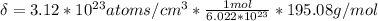Chemistry, 29.11.2019 05:31 ajayfurlow
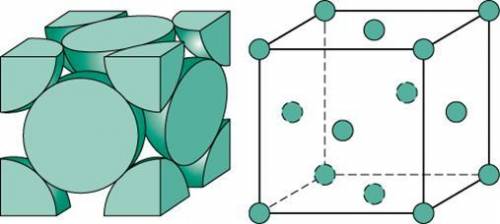
Ahypothetical metal crystallizes with the face-centered cubic unit cell. the radius of the metal atom is 234 picometers and its molar mass is 195.08 g/mol. calculate the density of the metal in g/cm3.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2


Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Ahypothetical metal crystallizes with the face-centered cubic unit cell. the radius of the metal ato...
Questions


English, 04.07.2019 13:40

English, 04.07.2019 13:40


Mathematics, 04.07.2019 13:40

English, 04.07.2019 13:40


English, 04.07.2019 13:40

Biology, 04.07.2019 13:40

Business, 04.07.2019 13:40


English, 04.07.2019 13:40


History, 04.07.2019 13:40






History, 04.07.2019 13:40