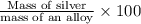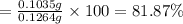Chemistry, 29.11.2019 05:31 SophieCasey
Asilver-copper alloy had a mass of 0.1264g. when the alloy was dissolved in nitric acid and the silver precipitated as silver chloride, the precipitate had a mass of 0.1375g. calculate the percent of silver in the alloy. for full credit, make sure you show your calculations.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

You know the right answer?
Asilver-copper alloy had a mass of 0.1264g. when the alloy was dissolved in nitric acid and the silv...
Questions



Mathematics, 23.03.2021 17:00

Mathematics, 23.03.2021 17:00



Mathematics, 23.03.2021 17:00


Mathematics, 23.03.2021 17:00

Chemistry, 23.03.2021 17:00

Health, 23.03.2021 17:00


English, 23.03.2021 17:00






History, 23.03.2021 17:00

Mathematics, 23.03.2021 17:00

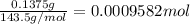
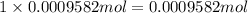 of silver
of silver