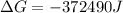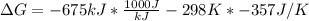Chemistry, 29.11.2019 06:31 Supermate11
Free-energy change, δg∘, is related to cell potential, e∘, by the equationδg∘=−nfe∘where n is the number of moles of electrons transferred and f=96,500c/(mol e−) is the faraday constant. when e∘ is measured in volts, δg∘ must be in joules since 1 j=1 c⋅v.1. calculate the standard free-energy change at 25 ∘c for the following reaction: mg(s)+fe2+(aq)→mg2+(aq)+fe(s)expres s your answer to three significant figures and include the appropriate units.2. calculate the standard cell potential at 25 ∘c for the reactionx(s)+2y+(aq)→x2+(aq)+2y(s)w here δh∘ = -675 kj and δs∘ = -357 j/k .express your answer to three significant figures and include the appropriate units.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Free-energy change, δg∘, is related to cell potential, e∘, by the equationδg∘=−nfe∘where n is the nu...
Questions

English, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30








Mathematics, 19.03.2021 20:30


Mathematics, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30


History, 19.03.2021 20:30

History, 19.03.2021 20:30



Mathematics, 19.03.2021 20:30

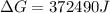

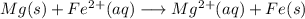
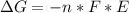
![E=E_{red}-E_{ox]](/tpl/images/0395/8514/34231.png)