
Sulfur dioxide, so2(g), can react with oxygen to produce sulfur trioxide, so3(g), by the following reaction
2so2+o2=2so3
the standard enthalpies of formation for so2(g) and so3(g) are
deltah= so2(g)= -296.8 kj
dh= so3(g)= -395.7 kj
calculate the amount of energy in the form of heat that is produced when a volume of 3.75 l of so2(g) is converted to 3.75 l of so3(g) according to this process at a constant pressure and temperature of 1.00 atm and 25.0

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1


Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
You know the right answer?
Sulfur dioxide, so2(g), can react with oxygen to produce sulfur trioxide, so3(g), by the following r...
Questions












History, 28.10.2019 22:31



Mathematics, 28.10.2019 22:31


Mathematics, 28.10.2019 22:31

History, 28.10.2019 22:31


Health, 28.10.2019 22:31

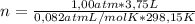 = 0,153 moles of reaction
= 0,153 moles of reaction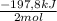 = -15,1kJ
= -15,1kJ

