Chemistry, 29.11.2019 06:31 cathydaves
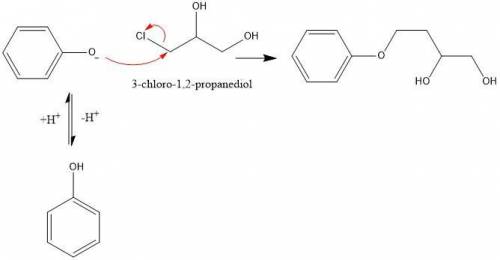
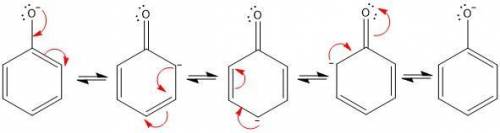
1. in the first step of the mechanism for this process, a phenoxide anion is generated. this phenoxide anion goes on to act as a nucleophile via an sn2 mechanism, displacing the chloride on 3-chloro-1,2-propanediol. why doesn’t the phenoxide anion act as a base to deprotonate one of the alcohols on 3-chloro-1,2-propanediol? write a brief, specific explanation (1-2 sentences).

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 09:30
Need , hurry pls create a superhero out of the element iron, what are its powers and his sidekick ( an element that works well with iron). how was the superhero made and who discovered him
Answers: 3

Chemistry, 23.06.2019 19:00
How can evidence from an experiment be explained in her relationship to the hypothesis? a.as a prediction b.as a question c.as an in inference d.as a conclusion
Answers: 2
You know the right answer?
1. in the first step of the mechanism for this process, a phenoxide anion is generated. this phenoxi...
Questions


Mathematics, 17.11.2020 19:50


English, 17.11.2020 19:50




Mathematics, 17.11.2020 19:50

History, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50

Biology, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50



English, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50