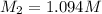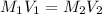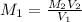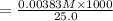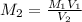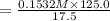Chemistry, 29.11.2019 06:31 sarahjdeering
A17.55 ml solution of potassium nitrate (kno3) was diluted to 125.0 ml, and 25.00 ml of this solution was then diluted to 1.000 × 103 ml. the concentration of the final solution is 0.00383 m. calculate the concentration of the original solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
A17.55 ml solution of potassium nitrate (kno3) was diluted to 125.0 ml, and 25.00 ml of this solutio...
Questions



English, 23.09.2021 02:00

Mathematics, 23.09.2021 02:00



Social Studies, 23.09.2021 02:00

Mathematics, 23.09.2021 02:00

Mathematics, 23.09.2021 02:00



Mathematics, 23.09.2021 02:00


Mathematics, 23.09.2021 02:00



Mathematics, 23.09.2021 02:00



Mathematics, 23.09.2021 02:00