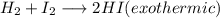Chemistry, 29.11.2019 07:31 heathkid23
 + i_2(g) \longrightarrow 2hi(g))
the forward reaction above is exothermic. at equilibrium, what happens if the reaction mixture is cooled at constant volume?
select all that apply.
1. the reaction absorbs energy.
2. the reaction releases energy.
3. [h₂] and [i₂] increase.
4. [h₂] and [i₂] decrease.
5. [h₂] and [i₂] remain constant.
6. [hi] increases.
7. [hi] decreases.
8. [hi] remains constant.
if c is added to the equilibrium system above, in which direction will the equilibrium shift?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
[tex]h_2(g) + i_2(g) \longrightarrow 2hi(g)[/tex]
the forward reaction above is exothermic. at...
the forward reaction above is exothermic. at...
Questions


Mathematics, 04.07.2019 09:40


Mathematics, 04.07.2019 09:40




Mathematics, 04.07.2019 09:40


Health, 04.07.2019 09:40

History, 04.07.2019 09:40

History, 04.07.2019 09:40

History, 04.07.2019 09:40


Mathematics, 04.07.2019 09:40

Mathematics, 04.07.2019 09:40



Mathematics, 04.07.2019 09:40

![[H_{2}]](/tpl/images/0395/9023/f6f78.png) and
and ![[I_{2}]](/tpl/images/0395/9023/d0626.png) increase.
increase.![[HI]](/tpl/images/0395/9023/7d1e5.png) increases.
increases.