
Chemistry, 30.11.2019 00:31 StacySams7361
Achemist combined chloroform ( chcl 3 ) and acetone ( c 3 h 6 o ) to create a solution where the mole fraction of chloroform, χ chloroform , is 0.203 . the densities of chloroform and acetone are 1.48 g/ml and 0.791 g/ml , respectively. calculate the molarity of the solution.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3


Chemistry, 23.06.2019 01:00
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
You know the right answer?
Achemist combined chloroform ( chcl 3 ) and acetone ( c 3 h 6 o ) to create a solution where the mol...
Questions


Mathematics, 30.05.2020 12:59

History, 30.05.2020 12:59


Biology, 30.05.2020 12:59

Physics, 30.05.2020 12:59

English, 30.05.2020 12:59



English, 30.05.2020 13:57




Mathematics, 30.05.2020 13:57

Geography, 30.05.2020 13:57

Biology, 30.05.2020 13:57


Health, 30.05.2020 13:57

History, 30.05.2020 13:57

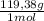 ×
×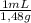 = 1637mL= 1,637L
= 1637mL= 1,637L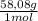 ×
×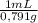 = 5852mL = 5,852L
= 5852mL = 5,852L

