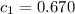Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
When a 40.0 g sample of a metal at 25.00 °c is added to 65.0 g of water at 100.00 °c, the final temp...
Questions

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Physics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40


Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40



Health, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Social Studies, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Biology, 12.12.2020 16:40


History, 12.12.2020 16:40

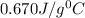
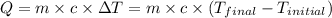
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0396/4522/09236.png) .................(1)
.................(1)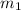 = mass of metal = 40.0 g
= mass of metal = 40.0 g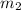 = mass of water = 65.0 g
= mass of water = 65.0 g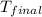 = final temperature =
= final temperature = 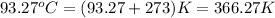
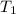 = temperature of metal =
= temperature of metal = 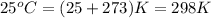
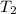 = temperature of water =
= temperature of water = 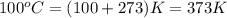
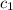 = specific heat of metal = ?
= specific heat of metal = ?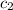 = specific heat of water=
= specific heat of water= 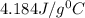
![40.0\times c_1\times (366.27-298)=-[65.0\times 4.184\times (366.27-373)]](/tpl/images/0396/4522/88e12.png)