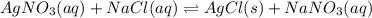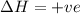Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
For the endothermic reaction below, using lechatlier's principle, predict which way the equilibrium...
Questions

Physics, 19.09.2019 00:30

History, 19.09.2019 00:30


Computers and Technology, 19.09.2019 00:30


History, 19.09.2019 00:30



Health, 19.09.2019 00:30

Mathematics, 19.09.2019 00:30

Mathematics, 19.09.2019 00:30







History, 19.09.2019 00:30