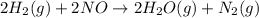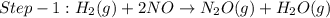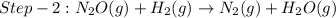Chemistry, 30.11.2019 01:31 uticabadgirl
The reduction of nitrogen monoxide is described by the following chemical equation: 2h2 (g) +2no (g) 2h20 ()n2 (g suppose a two-step mechanism is proposed for this reaction, beginning with this elementary reaction: h2 g+2no(g)- n20 (g)+h20(g) suppose also that the second step of the mechanism should be bimolecular suggest a reasonable second step. that is, write the balanced chemical equation of a bimolecular elementary reaction that would complete the proposed mechanism

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
The reduction of nitrogen monoxide is described by the following chemical equation: 2h2 (g) +2no (g...
Questions



Social Studies, 06.03.2021 08:00



Mathematics, 06.03.2021 08:00




Mathematics, 06.03.2021 08:00



Mathematics, 06.03.2021 08:00


Physics, 06.03.2021 08:00


English, 06.03.2021 08:00


Mathematics, 06.03.2021 08:00