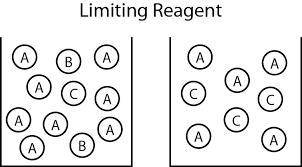
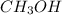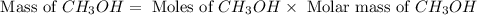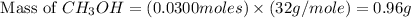Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: co(g)+2h2(g)→ch3oh(g) a 1.65 l reaction vessel, initially at 305 k, contains carbon monoxide gas at a partial pressure of 232 mmhg and hydrogen gas at a partial pressure of 374 mmhg .identify the limiting reactant and determine the theoretical yeild of methonal in grams.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: co(g)+2h2...
Questions



Biology, 09.07.2019 04:30


Physics, 09.07.2019 04:30




Mathematics, 09.07.2019 04:30



Social Studies, 09.07.2019 04:30


History, 09.07.2019 04:30


Biology, 09.07.2019 04:30

Social Studies, 09.07.2019 04:30

Biology, 09.07.2019 04:30

Computers and Technology, 09.07.2019 04:30

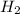 and the theoretical yield of methanol is, 0.96 grams.
and the theoretical yield of methanol is, 0.96 grams.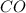 and
and 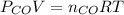
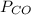 = pressure of CO gas = 232 mmHg = 0.305 atm (1 atm = 760 mmHg)
= pressure of CO gas = 232 mmHg = 0.305 atm (1 atm = 760 mmHg)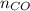 = number of moles of CO gas = ?
= number of moles of CO gas = ?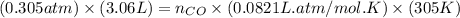
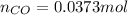
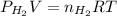
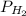 = pressure of
= pressure of 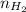 = number of moles of
= number of moles of 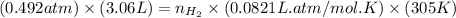
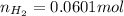
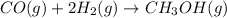
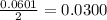 moles of
moles of