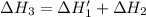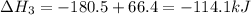Chemistry, 30.11.2019 01:31 tstaples02
Nitric acid can be manufactured in a multi-step process, during which nitric oxide is oxidized to create nitrogen dioxide. 2no (g) + o2 (g) → 2no2 (g)
calculate the standard reaction enthalpy for the above reaction using the following thermodynamic data.
n2 (g) + o2 (g) → 2no (g) ∆h˚1 = 180.5 kj
n2 (g) + 2o2 (g) → 2no2 (g) ∆h˚2 = 66.4 kj
-252.4 kj/mol rxn
-114.1 kj/mol rxn
-100.3 kj/mol rxn
-246.9 kj/mol rxn

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
Nitric acid can be manufactured in a multi-step process, during which nitric oxide is oxidized to cr...
Questions





Physics, 19.12.2019 06:31

Advanced Placement (AP), 19.12.2019 06:31

Mathematics, 19.12.2019 06:31

History, 19.12.2019 06:31


English, 19.12.2019 06:31

English, 19.12.2019 06:31

Chemistry, 19.12.2019 06:31




Mathematics, 19.12.2019 06:31

Mathematics, 19.12.2019 06:31

English, 19.12.2019 06:31


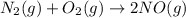 (1)
(1) 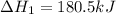
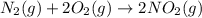 (2)
(2) 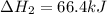
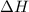 for the following reaction i.e,
for the following reaction i.e,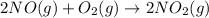 (3)
(3) 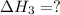
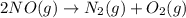 (1')
(1') 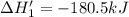
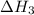 for the reaction will be:
for the reaction will be: