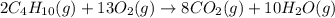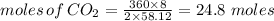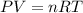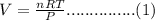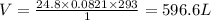Chemistry, 30.11.2019 02:31 mbatton879
Combustion of hydrocarbons such as butane () produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.1. write a balanced chemical equation, including physical state symbols, for the combustion of gaseous butane into gaseous carbon dioxide and gaseous water.2. suppose 0.360 kg of butane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 °c. calculate the volume of carbon dioxide gas that is produced be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1
You know the right answer?
Combustion of hydrocarbons such as butane () produces carbon dioxide, a "greenhouse gas." greenhouse...
Questions



Chemistry, 18.09.2021 09:10

English, 18.09.2021 09:10




English, 18.09.2021 09:10


Mathematics, 18.09.2021 09:10

Biology, 18.09.2021 09:10

Chemistry, 18.09.2021 09:10

Spanish, 18.09.2021 09:10


Law, 18.09.2021 09:10