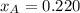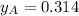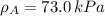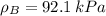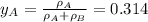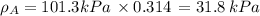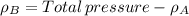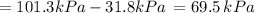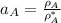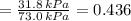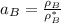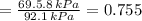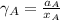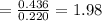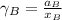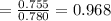By measuring the equilibrium between liquid and vapour phases of a solution at 30°c at 1.00 atm, it was found that xa = 0.220 when ya = 0.314. calculate the activities and activity coefficients of both components in this solution on the raoult’s law basis. the vapour pressures of the pure components at this temperature are: pa = 73.0 kpa and pb = 92.1 kpa. (xa is the mole fraction in the liquid and ya the mole fraction in the vapour.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

You know the right answer?
By measuring the equilibrium between liquid and vapour phases of a solution at 30°c at 1.00 atm, it...
Questions

Chemistry, 02.06.2021 02:40

Mathematics, 02.06.2021 02:40


Social Studies, 02.06.2021 02:40



Mathematics, 02.06.2021 02:40


English, 02.06.2021 02:40



Mathematics, 02.06.2021 02:40



Mathematics, 02.06.2021 02:40

Physics, 02.06.2021 02:40

Physics, 02.06.2021 02:50


Chemistry, 02.06.2021 02:50

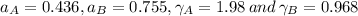 .
.