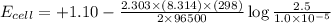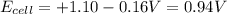The standard cell potential (e°cell) for the reaction below is +1.10v. the cell potential for this reaction is v when the concentration of [cu2+]=1.0⋅10−5m and [zn2+]=2.5m. zn (s) + cu2+ (aq) → cu (s) + zn2+ (aq) the standard cell potential () for the reaction below is . the cell potential for this reaction is when the concentration of and (s) + (aq) (s) + (aq) 0.78 1.10 0.94 1.26 1.42

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
The standard cell potential (e°cell) for the reaction below is +1.10v. the cell potential for this r...
Questions


Mathematics, 11.03.2021 19:10

Biology, 11.03.2021 19:10


Mathematics, 11.03.2021 19:10



Mathematics, 11.03.2021 19:10

Spanish, 11.03.2021 19:10


English, 11.03.2021 19:10

Biology, 11.03.2021 19:10

Mathematics, 11.03.2021 19:10

English, 11.03.2021 19:10

Arts, 11.03.2021 19:10


Mathematics, 11.03.2021 19:10



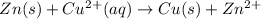
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]}{[Cu^{2+}]}](/tpl/images/0396/8274/2e6f5.png)
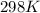
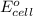 = standard electrode potential of the cell = +1.10 V
= standard electrode potential of the cell = +1.10 V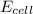 = emf of the cell = ?
= emf of the cell = ?