
Chemistry, 30.11.2019 03:31 emily200705
If the equilibrium constant for a reaction is 0.00010, what does this tell us about the position of equilibrium for that reaction?
a. there are more reactants than products at equilibrium.
b. there are more products than reactants at equilibrium.
c. at equilibrium, the concentration of reactants is the same as that of the products.
d. the concentration of the product at equilibrium is 0.00010 m.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

You know the right answer?
If the equilibrium constant for a reaction is 0.00010, what does this tell us about the position of...
Questions


Mathematics, 18.02.2021 02:00




Biology, 18.02.2021 02:00




Geography, 18.02.2021 02:00



Mathematics, 18.02.2021 02:00

Mathematics, 18.02.2021 02:00

Mathematics, 18.02.2021 02:00

Mathematics, 18.02.2021 02:00

Spanish, 18.02.2021 02:00

Health, 18.02.2021 02:00

Social Studies, 18.02.2021 02:00

Mathematics, 18.02.2021 02:00

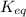
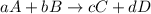
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0396/7601/9c8b0.png)
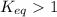 ; the reaction is product favored.
; the reaction is product favored.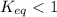 ; the reaction is reactant favored.
; the reaction is reactant favored.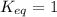 ; the reaction is in equilibrium.
; the reaction is in equilibrium.

