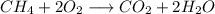Chemistry, 30.11.2019 04:31 wcraig1998
Arigid stainless steel chamber contains 140 torr of methane, ch4, and excess oxygen, o2, at 160.0 °c. a spark is ignited inside the chamber, completely combusting the methane. what is the change in total pressure within the chamber following the reaction? assume a constant temperature throughout the process.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

You know the right answer?
Arigid stainless steel chamber contains 140 torr of methane, ch4, and excess oxygen, o2, at 160.0 °c...
Questions


Spanish, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30

French, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30





Mathematics, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30

English, 02.03.2021 07:30

Health, 02.03.2021 07:30

English, 02.03.2021 07:30

English, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30

Social Studies, 02.03.2021 07:30