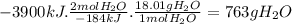Chemistry, 30.11.2019 05:31 HistoryLee
According to the following thermochemical equation, what mass of h2o (in g) must form in order to produce 3900 kj of energy?
sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh°rxn = -184 kj sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh°rxn = -184 kj 216 g 382 g 763 g 408 g 272 g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

You know the right answer?
According to the following thermochemical equation, what mass of h2o (in g) must form in order to pr...
Questions


Mathematics, 17.04.2021 06:30

English, 17.04.2021 06:30

Mathematics, 17.04.2021 06:30

History, 17.04.2021 06:30

Mathematics, 17.04.2021 06:30

Mathematics, 17.04.2021 06:30

Mathematics, 17.04.2021 06:30

Mathematics, 17.04.2021 06:30


History, 17.04.2021 06:30

Chemistry, 17.04.2021 06:30

Mathematics, 17.04.2021 06:30


Mathematics, 17.04.2021 06:30


Mathematics, 17.04.2021 06:30

Mathematics, 17.04.2021 06:30


Chemistry, 17.04.2021 06:30