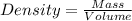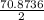Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 14:00
Total number of electrons that can occupy the d sub level
Answers: 1
You know the right answer?
An unknown diatomic gas has a density of 3.164 g/l at stp. what is the identity of the gas?...
Questions


Mathematics, 09.07.2019 12:30

History, 09.07.2019 12:30








Mathematics, 09.07.2019 12:30

Mathematics, 09.07.2019 12:30

Mathematics, 09.07.2019 12:30

Mathematics, 09.07.2019 12:30



Mathematics, 09.07.2019 12:30

Mathematics, 09.07.2019 12:30

Chemistry, 09.07.2019 12:30

Mathematics, 09.07.2019 12:30