
Chemistry, 30.11.2019 05:31 rubyhart522
Calcium chloride, cacl2, is commonly used as an electrolyte in sports drinks and other beverages, including bottled water. a solution is made by adding 6.50 g of cacl2 to 60.0 ml of water at 25∘c. the density of the solvent at that temperature is 0.997 g/ml. calculate the mole percent of cacl2 in the solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
Calcium chloride, cacl2, is commonly used as an electrolyte in sports drinks and other beverages, in...
Questions


Mathematics, 20.04.2021 17:30

English, 20.04.2021 17:30



Biology, 20.04.2021 17:30

Chemistry, 20.04.2021 17:30


Mathematics, 20.04.2021 17:30

English, 20.04.2021 17:30




Chemistry, 20.04.2021 17:30


English, 20.04.2021 17:30

English, 20.04.2021 17:30


Social Studies, 20.04.2021 17:30

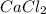 in solution is 1.71%
in solution is 1.71%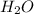 = 18.02 g/mol
= 18.02 g/mol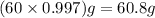
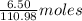 of
of 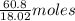 of
of 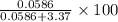 % = 1.71%
% = 1.71%

