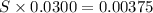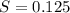What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is required to completely react with 25.00 ml of a 0.300 m thiosulfate solution, k2s2o3(aq)? the chemical equation for the reaction is 2 s2o32-(aq) + i3-(aq) → s4o62-(aq) + 3 i-(aq).

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1


Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3
You know the right answer?
What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is requ...
Questions



Geography, 14.11.2020 21:30

Mathematics, 14.11.2020 21:30


English, 14.11.2020 21:30

Mathematics, 14.11.2020 21:30

English, 14.11.2020 21:30


Mathematics, 14.11.2020 21:30


Mathematics, 14.11.2020 21:30

Arts, 14.11.2020 21:30





Health, 14.11.2020 21:30


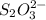 completely react with 1 mol of
completely react with 1 mol of 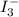 .
.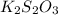 solution =
solution = 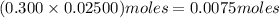
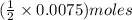 of
of 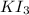 solution is S (M) then-
solution is S (M) then-