
Chemistry, 30.11.2019 06:31 Virnalis1112
What is the [h3o+] and the ph of a benzoic acid-benzoate buffer that consists of 0.17 m c6h5cooh and 0.42 m c6h5coona? (ka of benzoic acid = 6.3 × 10^−5). be sure to report your answer to the correct number of significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 20.06.2019 18:04
According to the balanced chemical equation 5 h2c2o4(aq) + 2 mno4-(aq) + 6 h+(aq) → 10 co2(g) + 2 mn2+(aq) + 8 h2o(l) 0.875 grams of oxalic acid, h2c2o4 will react with moles of permanganate, mno4-.
Answers: 2

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
What is the [h3o+] and the ph of a benzoic acid-benzoate buffer that consists of 0.17 m c6h5cooh and...
Questions

Computers and Technology, 19.09.2019 10:30



Mathematics, 19.09.2019 10:30

Mathematics, 19.09.2019 10:30



Social Studies, 19.09.2019 10:30

Mathematics, 19.09.2019 10:30


Mathematics, 19.09.2019 10:30



Chemistry, 19.09.2019 10:30



History, 19.09.2019 10:30



Mathematics, 19.09.2019 10:30

![[H_{3}O^{+}]=x M = 2.5\times 10^{-5}M](/tpl/images/0397/1268/ab31e.png) and pH = 4.6
and pH = 4.6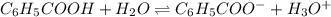
![\frac{[C_{6}H_{5}COO^{-}][H_{3}O^{+}]}{[C_{6}H_{5}COOH]}=K_{a}(C_{6}H_{5}COOH)](/tpl/images/0397/1268/75106.png)
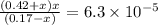
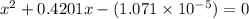
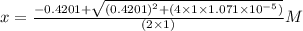
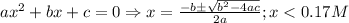 )
)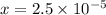 M
M![pH=-log[H_{3}O^{+}]=-logx=-log(2.5\times 10^{-5})=4.6](/tpl/images/0397/1268/f0ff1.png)


