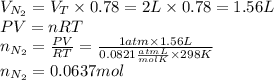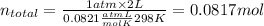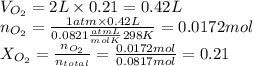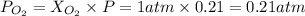An "empty" container is not really empty if it contains air. how may moles of nitrogen are in an "empty" two-liter cola bottle at atmospheric pressure and room temperature (25∘c)? assume ideal behavior. what is the partial pressure of oxygen in air at atmospheric pressure (1 atm)? assume ideal behavior

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2


Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
An "empty" container is not really empty if it contains air. how may moles of nitrogen are in an "em...
Questions


Mathematics, 02.04.2020 02:00



Mathematics, 02.04.2020 02:00

Mathematics, 02.04.2020 02:00

English, 02.04.2020 02:00

Mathematics, 02.04.2020 02:00


Mathematics, 02.04.2020 02:01

Mathematics, 02.04.2020 02:01



Chemistry, 02.04.2020 02:01