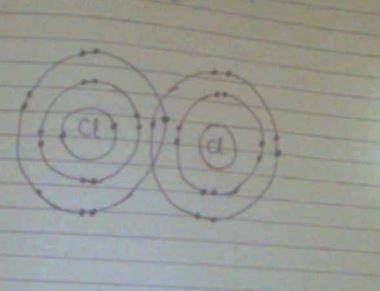
Where are the electrons most probably located in a molecular bonding orbital?
a. in stationar...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3



Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
Questions

Mathematics, 15.05.2021 21:00

Mathematics, 15.05.2021 21:00

Mathematics, 15.05.2021 21:00


Mathematics, 15.05.2021 21:00


Mathematics, 15.05.2021 21:00


History, 15.05.2021 21:00




Mathematics, 15.05.2021 21:00

Mathematics, 15.05.2021 21:00

Mathematics, 15.05.2021 21:00


Physics, 15.05.2021 21:00

Spanish, 15.05.2021 21:10

Mathematics, 15.05.2021 21:10