
An element crystallizes in a face centered cubic lattice and has a density of 1.45 g cm-3 . the edge of its unit cell is 4.52 x 10-8cm.
a) how many atoms are in each unit cell?
b) what is the volume of a unit cell?
c) what is the mass of a unit cell?
d) calculate the approximate atomic mass of the element.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
An element crystallizes in a face centered cubic lattice and has a density of 1.45 g cm-3 . the edge...
Questions



Mathematics, 13.07.2020 20:01

Physics, 13.07.2020 20:01

Spanish, 13.07.2020 20:01





Computers and Technology, 13.07.2020 20:01


Mathematics, 13.07.2020 20:01

Spanish, 13.07.2020 20:01


Mathematics, 13.07.2020 20:01


Mathematics, 13.07.2020 20:01


Mathematics, 13.07.2020 20:01

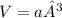 , a being the edge length.
, a being the edge length.


