
Chemistry, 30.11.2019 07:31 kitttimothy55
The heat of vaporization of water at 100°c is 40.66 kj/mol. calculate the quantity of heat that is absorbed/released when 9.00 g of steam condenses to liquid water at 100°c.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

You know the right answer?
The heat of vaporization of water at 100°c is 40.66 kj/mol. calculate the quantity of heat that is a...
Questions

Mathematics, 08.10.2019 07:10

Advanced Placement (AP), 08.10.2019 07:10


Mathematics, 08.10.2019 07:10


Mathematics, 08.10.2019 07:10

History, 08.10.2019 07:10


Physics, 08.10.2019 07:10


Mathematics, 08.10.2019 07:10

Mathematics, 08.10.2019 07:10

Mathematics, 08.10.2019 07:10

Biology, 08.10.2019 07:10



Chemistry, 08.10.2019 07:10

Mathematics, 08.10.2019 07:10

English, 08.10.2019 07:10

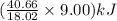 of heat or 20.3 kJ of heat is being consumed
of heat or 20.3 kJ of heat is being consumed

