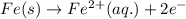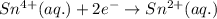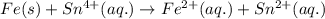Chemistry, 30.11.2019 07:31 vanessa051266
In the electrochemical cell using the redox reaction below, the anode half reaction is sn4+ (aq) + fe (s) → sn2+ (aq) + fe2+ (aq) in the electrochemical cell using the redox reaction below, the anode half reaction is (aq) + (s) (aq) + (aq) fe→fe2++2e− sn4+→sn2++2e− fe+2e−→fe2+ sn4++2e−→sn2+ fe+2e−→sn2+ request answer

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 12:50
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1

Chemistry, 23.06.2019 14:20
What kind of chemical reaction does the chemical equation sodium + chlorine → sodium chloride represent? a. combustion b. decomposition c. single replacement d. synthesis
Answers: 1

Chemistry, 23.06.2019 19:00
Hjulstrom's diagram plots two curves representing (1) the minimum stream velocity required to erode sediments of varying sizes from the stream bed, and (2) the minimum velocity required to continue to transport sediments of varying sizes. this is a modified version of the diagram, showing only the transport segment. stream competence refers to the heaviest particles a stream can carry. according to hjulstrom's diagram stream competence depends on a) particle size. b) stream velocity. c) stream deposition. d) stream channel shape.
Answers: 2

Chemistry, 23.06.2019 20:30
Due tomorrow write the chemical equation that has the equilibrium constant expression [listed in photo]
Answers: 2
You know the right answer?
In the electrochemical cell using the redox reaction below, the anode half reaction is sn4+ (aq) +...
Questions


Mathematics, 25.11.2020 17:10

Chemistry, 25.11.2020 17:10

Mathematics, 25.11.2020 17:10


World Languages, 25.11.2020 17:10


Mathematics, 25.11.2020 17:10

Physics, 25.11.2020 17:10


Mathematics, 25.11.2020 17:10

Mathematics, 25.11.2020 17:10



Advanced Placement (AP), 25.11.2020 17:10

Physics, 25.11.2020 17:10


History, 25.11.2020 17:10