
Chemistry, 01.12.2019 00:31 hei40563273
8. select the lattice energy for rubidium chloride from the following data (in kj/mol]
rb(s) > rb(g) = 85.8
ie1(rb) = 397.5
be(cl2) = 226
deltahf(rbcl) = -431
electron affinity cl = -332
a. -53.7
b. +53.7
c. -695
d. -808
e. +808

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
8. select the lattice energy for rubidium chloride from the following data (in kj/mol]
rb(s) &g...
rb(s) &g...
Questions

Mathematics, 04.09.2020 14:01




English, 04.09.2020 14:01


Business, 04.09.2020 14:01

Computers and Technology, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01


Mathematics, 04.09.2020 14:01

History, 04.09.2020 14:01







History, 04.09.2020 14:01

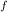 = -431 kJ/mol
= -431 kJ/mol![8. select the lattice energy for rubidium chloride from the following data (in kj/mol]rb(s) > rb](/tpl/images/0397/7697/0aca5.jpg)


