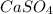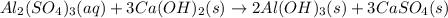Given the unbalanced equation:
- al(so )3 + - ca(oh)2 +_ al(oh)3 + caso,
what is the coef...

Chemistry, 01.12.2019 09:31 trinityparrish47
Given the unbalanced equation:
- al(so )3 + - ca(oh)2 +_ al(oh)3 + caso,
what is the coefficient in front of the caso, when the equation is completely balanced with
the smallest whole-number coefficients?
a. 1
b.2
c.3
d.4

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
Questions


Mathematics, 30.05.2020 04:59






Mathematics, 30.05.2020 04:59

History, 30.05.2020 04:59

Biology, 30.05.2020 04:59

Mathematics, 30.05.2020 04:59




English, 30.05.2020 04:59

Mathematics, 30.05.2020 04:59


Computers and Technology, 30.05.2020 04:59

Mathematics, 30.05.2020 04:59

Mathematics, 30.05.2020 04:59