Draw lewis structures for each of the following.
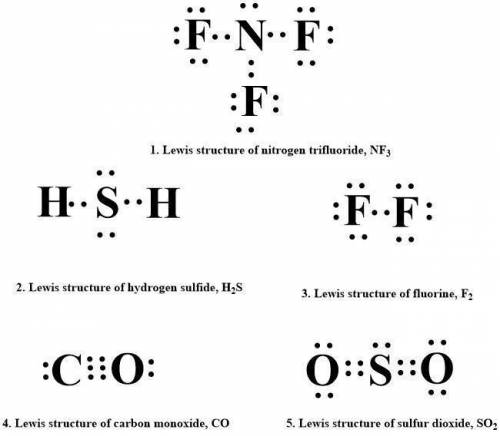
1. nitrogen trifluoride, nf3
2. hydrogen sulfide, h2s
3. fluorine, f2
4. carbon monoxide, co
5. sulfur dioxide, so2
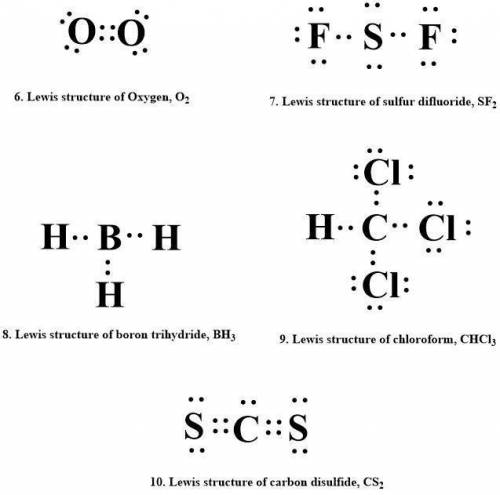
6. oxygen, o2
7. sulfur difluoride, sf2
8. boron trihydride, bhz
9. chloroform, chcl3
10. carbon disulfide, cs2
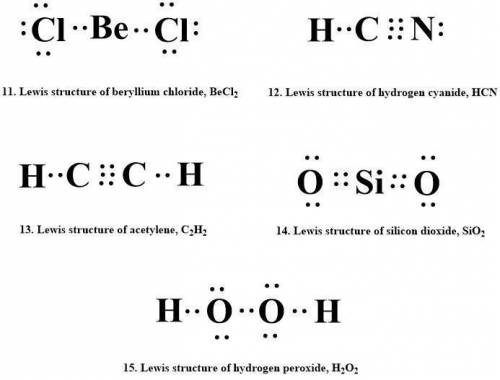
11. beryllium chloride, becl2
12. hydrogen cyanide, hcn
13. acetylene, c2h2
14. silicon dioxide, sio2
15. hydrogen peroxide, h2o2
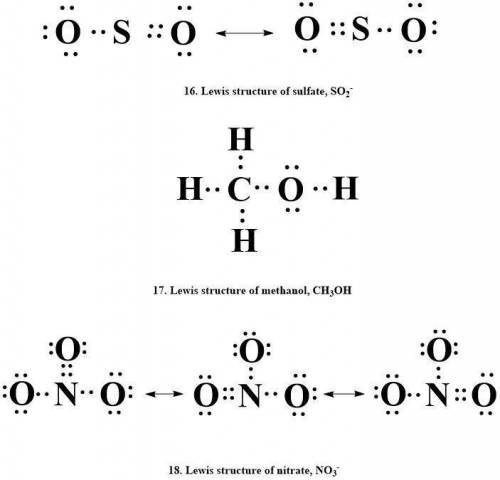
16. sulfate, so2-
17. methanol, ch3oh
18. nitrate, no3
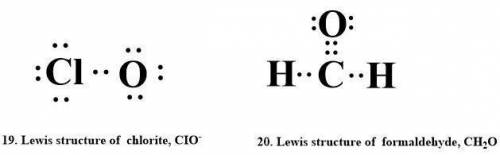
19. chlorite, cio,
20. formic acid, ch2o

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1


You know the right answer?
Draw lewis structures for each of the following.
1. nitrogen trifluoride, nf3
2. hydroge...
1. nitrogen trifluoride, nf3
2. hydroge...
Questions

Mathematics, 28.06.2019 19:40

Mathematics, 28.06.2019 19:40

Physics, 28.06.2019 19:40

History, 28.06.2019 19:40

History, 28.06.2019 19:40


Chemistry, 28.06.2019 19:40

Mathematics, 28.06.2019 19:40

Mathematics, 28.06.2019 19:40

Mathematics, 28.06.2019 19:40



Mathematics, 28.06.2019 19:40


Spanish, 28.06.2019 19:40

History, 28.06.2019 19:40


Mathematics, 28.06.2019 19:40