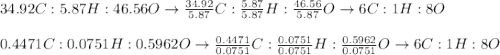Chemistry, 02.12.2019 15:31 ryleerose255
Asample of glucose is found to have 34.92 g of carbon, 5.87 g of hydrogen and 46.56 g of oxygen. another sample is found to have 0.4471 g of carbon, 0.07510 g of hydrogen, and 0.5962 g of oxygen. show that these results are consistent with the law of definite proportions.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
You know the right answer?
Asample of glucose is found to have 34.92 g of carbon, 5.87 g of hydrogen and 46.56 g of oxygen. ano...
Questions


Health, 26.07.2019 05:00





Mathematics, 26.07.2019 05:00

Mathematics, 26.07.2019 05:00

Biology, 26.07.2019 05:00


Spanish, 26.07.2019 05:00

Biology, 26.07.2019 05:00


Biology, 26.07.2019 05:00

Biology, 26.07.2019 05:00





Mathematics, 26.07.2019 05:00