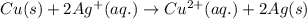Chemistry, 02.12.2019 18:31 hayesvolcano
Write the half-reactions as they occur at each electrode and the net cell reaction for this electrochemical cell containing copper and silver. cu ( s ) ∣ ∣ cu 2 + ( aq ) ∥ ∥ ag + ( aq ) ∣ ∣ ag ( s ) anode: cathode: net cell reaction:

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
Write the half-reactions as they occur at each electrode and the net cell reaction for this electroc...
Questions

Mathematics, 26.05.2021 16:20

Spanish, 26.05.2021 16:20

Mathematics, 26.05.2021 16:20


History, 26.05.2021 16:20



Biology, 26.05.2021 16:20


Geography, 26.05.2021 16:20

English, 26.05.2021 16:20

Mathematics, 26.05.2021 16:20



Mathematics, 26.05.2021 16:20

Physics, 26.05.2021 16:20


Mathematics, 26.05.2021 16:20

Spanish, 26.05.2021 16:20

Mathematics, 26.05.2021 16:20

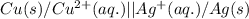
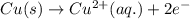
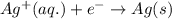 ( × 2)
( × 2)