
Chemistry, 02.12.2019 19:31 elitehairnerd1964
When 100 ml of 1.0 m na3po4 is mixed with 100 ml of 1.0 m agno3,
a yellow precipitate forms and ag+ becomes negligibly small. which
of the following is the correct listing of the ions remaining in solution
in order of increasing concentration?
(a) po43- < no3- < na+
(b) po43- < na+ < no3-
(c) no3- < po43- < na+
(d) na+ < no3- < po43-
(e) na+ < po43- < no3-

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
When 100 ml of 1.0 m na3po4 is mixed with 100 ml of 1.0 m agno3,
a yellow precipitate fo...
a yellow precipitate fo...
Questions

Computers and Technology, 10.09.2019 07:20

Mathematics, 10.09.2019 07:20

History, 10.09.2019 07:20

Mathematics, 10.09.2019 07:20

Mathematics, 10.09.2019 07:20

Mathematics, 10.09.2019 07:20



History, 10.09.2019 07:20

Mathematics, 10.09.2019 07:20




Mathematics, 10.09.2019 07:20

Mathematics, 10.09.2019 07:20



Mathematics, 10.09.2019 07:20

Mathematics, 10.09.2019 07:20

Chemistry, 10.09.2019 07:20

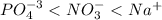
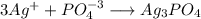
![[Ag^+]=0 M](/tpl/images/0399/5580/6ee64.png)
![[PO_4^{-3}]=0.5 M-0.5 M \frac{1 mol PO4}{3 mol Ag}=0.33 M](/tpl/images/0399/5580/8a590.png)
![[Na^+]=0.5 M * \frac{3 mol Na}{mol Na_3PO_4}=1.5 M](/tpl/images/0399/5580/07524.png)
![[NO_3^-]=0.5 M](/tpl/images/0399/5580/9ee3a.png)


