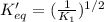Chemistry, 02.12.2019 21:31 heatherswiffin666
The reaction: 2 so2(g) + o2(g) --> 2 so3(g) has an equilibrium constant of k1. what is the k value for the reaction: so3(g) --> so2(g) + ½ o2(g)?
k1^½
1/k1
½ k1
(1/k1)^½

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
The reaction: 2 so2(g) + o2(g) --> 2 so3(g) has an equilibrium constant of k1. what is the k va...
Questions




Business, 04.07.2019 23:40

History, 04.07.2019 23:40

Biology, 04.07.2019 23:40

Social Studies, 04.07.2019 23:40

Social Studies, 04.07.2019 23:50


History, 04.07.2019 23:50



Business, 04.07.2019 23:50

Mathematics, 04.07.2019 23:50



Biology, 04.07.2019 23:50

History, 04.07.2019 23:50


Biology, 04.07.2019 23:50

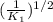
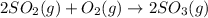
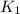
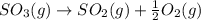
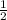 ', the equilibrium constant of the reverse reaction will be the 1/2 power of the equilibrium constant of initial reaction.
', the equilibrium constant of the reverse reaction will be the 1/2 power of the equilibrium constant of initial reaction.