
Chemistry, 02.12.2019 21:31 krystalhurst97
Using the standard enthalpies of formation for the chemicals involved, calculate the enthalpy change for the following reaction.
(note: show the math clearly and provide units in your set up) ( hf values in kj/mol are as follows: no2 32, h2o 286, hno3 207, no 90.)
3no2(g) h2o(l) 2hno3(aq) no(g) g

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
Using the standard enthalpies of formation for the chemicals involved, calculate the enthalpy change...
Questions

Mathematics, 29.08.2020 18:01

Mathematics, 29.08.2020 18:01


Chemistry, 29.08.2020 18:01


Advanced Placement (AP), 29.08.2020 18:01







Health, 29.08.2020 18:01




Mathematics, 29.08.2020 18:01

English, 29.08.2020 18:01

Mathematics, 29.08.2020 18:01

Mathematics, 29.08.2020 18:01

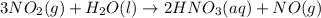
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0399/7869/76c37.png)
![\Delta H=[(n_{HNO_3}\times \Delta H_{HNO_3})+(n_{NO}\times \Delta H_{NO})]-[(n_{H_2O}\times \Delta H_{H_2O})+(n_{NO_2}\times \Delta H_{NO_2})]](/tpl/images/0399/7869/7081c.png)
![\Delta H=[(2\times -207)+(1\times 90)]-[(1\times -286)+(3\times 32)]](/tpl/images/0399/7869/1d6ad.png)



