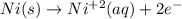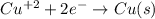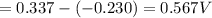Chemistry, 02.12.2019 21:31 loveashley1
Part a describe the electrodes in this nickel-copper galvanic cell. drag the appropriate items to their respective bins. view available hint(s) nickel copper standard reduction potentials for nickel(ii) and copper(ii) the standard reduction potential for a substance indicates how readily that substance gains electrons relative to other substances at standard conditions. the more positive the reduction potential, the more easily the substance gains electrons. consider the following: ni2+(aq)+2e−→ni(s),cu2+(aq)+2e−→cu( s), e∘red=−0.230 v e∘red=+0.337 v part b what is the standard potential, e∘cell, for this galvanic cell? use the given standard reduction potentials in your calculation as appropriate.

Answers: 3
Another question on Chemistry




Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
Part a describe the electrodes in this nickel-copper galvanic cell. drag the appropriate items to th...
Questions

Social Studies, 03.08.2019 21:30


Business, 03.08.2019 21:30


Biology, 03.08.2019 21:30

Mathematics, 03.08.2019 21:30


Computers and Technology, 03.08.2019 21:30

History, 03.08.2019 21:30


History, 03.08.2019 21:30

Mathematics, 03.08.2019 21:30


Social Studies, 03.08.2019 21:30

Geography, 03.08.2019 21:30





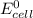 of the reaction is 0.567V.
of the reaction is 0.567V.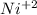 solution.
solution. 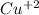 solution.
solution.