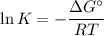Chemistry, 02.12.2019 22:31 raymondmancilla123
What value of the equilibrium constant, k, at 25ºc corresponds to a large negative value of δgº?
small negative value
small positive value
large negative value
large positive value

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
What value of the equilibrium constant, k, at 25ºc corresponds to a large negative value of δgº?
Questions



Mathematics, 28.08.2019 12:30


Social Studies, 28.08.2019 12:30






History, 28.08.2019 12:30





Social Studies, 28.08.2019 12:30




English, 28.08.2019 12:30