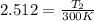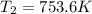Chemistry, 02.12.2019 22:31 mommy4life678
An ideal diatomic gas starting at room temperature t1 = 300 k and atmospheric pressure p1 = 1.0 atm is compressed adiabatically to 1/10 of its original volume. what is the final temperature of the gas?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
An ideal diatomic gas starting at room temperature t1 = 300 k and atmospheric pressure p1 = 1.0 atm...
Questions


Social Studies, 26.12.2019 09:31

English, 26.12.2019 09:31


Business, 26.12.2019 09:31

Mathematics, 26.12.2019 09:31

Business, 26.12.2019 09:31

English, 26.12.2019 09:31

Mathematics, 26.12.2019 09:31


Mathematics, 26.12.2019 09:31

History, 26.12.2019 09:31


Chemistry, 26.12.2019 09:31

History, 26.12.2019 09:31

Mathematics, 26.12.2019 09:31

History, 26.12.2019 09:31

Mathematics, 26.12.2019 09:31


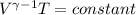
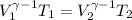
![\left [\frac{V_{1}}{V_{2}} \right ]^{\gamma-1 } = \frac{T_{2}}{T_{1}}](/tpl/images/0399/8638/7957d.png)
![\left [10 \right ]^{\frac{7}{5}-1 } = \frac{T_{2}}{300 K}](/tpl/images/0399/8638/fd268.png)
![\left [10 \right ]^{\frac{2}{5} } = \frac{T_{2}}{300 K}](/tpl/images/0399/8638/77b45.png)