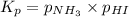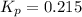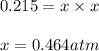Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide. nh4i (s) ⇌ nh3 (g) + hi (g) at 400 ºc, kp = 0.215. calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400 ºc. complete the ice box below as part of your answer.
a. 0.103 atmb. 0.215 atmc. 0.232 atmd. 0.464 atme. 2.00 atm

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1
You know the right answer?
Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide. nh4i (s) ⇌ nh3 (g) + hi (g) a...
Questions


Physics, 24.11.2020 02:00



English, 24.11.2020 02:00


Engineering, 24.11.2020 02:00



History, 24.11.2020 02:00

Arts, 24.11.2020 02:00

Mathematics, 24.11.2020 02:00

Mathematics, 24.11.2020 02:00


Mathematics, 24.11.2020 02:00


Social Studies, 24.11.2020 02:00

Social Studies, 24.11.2020 02:00


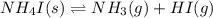
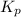 for the following equation is:
for the following equation is: