
Chemistry, 02.12.2019 23:31 tmrodriguez1
At 25°c, the standard enthalpy of combustion of gaseous propane (c3h8) is –2219.0 kj per mole of propane, and the standard enthalpy of combustion of gaseous propylene (c3h6) is –2058.3 kj per mole of propylene.
what is the standard enthalpy change for the following reaction at 25°c? c3h6(g) + h2(g) → c3h8(g)substance∆h°f (kj/mol)co2(g)–393.5h2o(l)–285.8

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1

Chemistry, 23.06.2019 14:00
Total number of electrons that can occupy the d sub level
Answers: 1
You know the right answer?
At 25°c, the standard enthalpy of combustion of gaseous propane (c3h8) is –2219.0 kj per mole of pro...
Questions


English, 24.08.2019 22:10

Chemistry, 24.08.2019 22:10

Mathematics, 24.08.2019 22:10

Social Studies, 24.08.2019 22:10

Mathematics, 24.08.2019 22:10

Mathematics, 24.08.2019 22:10

History, 24.08.2019 22:10


Mathematics, 24.08.2019 22:10

Mathematics, 24.08.2019 22:10

Mathematics, 24.08.2019 22:10

Mathematics, 24.08.2019 22:10



English, 24.08.2019 22:10

Mathematics, 24.08.2019 22:10



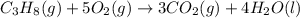
![\Delta H^o_{rxn}=[(3\times \Delta H^o_f_{(CO_2(g))})+(4\times \Delta H^o_f_{(H_2O(g))})]-[(1\times \Delta H^o_f_{(C_3H_8(g))})+(5\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0399/9365/6eb42.png)
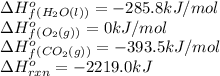
![-2219.0=[(3\times (-393.5))+(4\times (-285.8))]-[(1\times \Delta H^o_f_{(C_3H_8(g))})+(5\times (0))]\\\\\Delta H^o_f_{(C_3H_8(g))}=-140.7kJ/mol](/tpl/images/0399/9365/987c0.png)
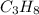 is -140.7 kJ/mol
is -140.7 kJ/mol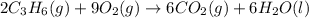
![\Delta H^o_{rxn}=[(6\times \Delta H^o_f_{(CO_2(g))})+(6\times \Delta H^o_f_{(H_2O(g))})]-[(2\times \Delta H^o_f_{(C_3H_6(g))})+(9\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0399/9365/b6201.png)
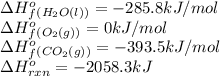
![-2058.3=[(6\times (-393.5))+(6\times (-285.8))]-[(2\times \Delta H^o_f_{(C_3H_6(g))})+(9\times (0))]\\\\\Delta H^o_f_{(C_3H_6(g))}=-1008.75kJ/mol](/tpl/images/0399/9365/ecedb.png)
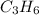 is -1008.75 kJ/mol
is -1008.75 kJ/mol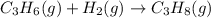
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(C_3H_8(g))})]-[(1\times \Delta H^o_f_{(C_3H_6(g))})+(1\times \Delta H^o_f_{(H_2(g))})]](/tpl/images/0399/9365/9b762.png)
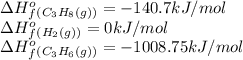
![\Delta H^o_{rxn}=[(1\times (-140.7))]-[(1\times (-1008.75))+(1\times (0))]\\\\\Delta H^o_{rxn}=868.05kJ](/tpl/images/0399/9365/ff366.png)


