

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
Given thath2(g) + f2(g) -> 2hf(g) => ∆h = -546.6 kj . mol-12h2(g) + o2(g) -> 2h20(l) =&g...
Questions


Health, 26.01.2021 20:30

Physics, 26.01.2021 20:30


English, 26.01.2021 20:30


Business, 26.01.2021 20:30

Mathematics, 26.01.2021 20:30

Biology, 26.01.2021 20:30


Computers and Technology, 26.01.2021 20:30


Mathematics, 26.01.2021 20:30

English, 26.01.2021 20:30


History, 26.01.2021 20:30


Mathematics, 26.01.2021 20:30

History, 26.01.2021 20:30

Mathematics, 26.01.2021 20:30

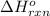 for the reaction is -521.6 kJ.
for the reaction is -521.6 kJ.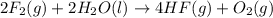
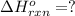
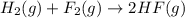
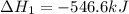 ( × 2)
( × 2)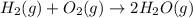
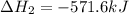
![\Delta H^o_{rxn}=[2\times \Delta H_1]+[1\times (-\Delta H_2)]](/tpl/images/0400/0579/648b7.png)
![\Delta H^o_{rxn}=[(2\times (-546.6))+(1\times (571.6))]=-521.6kJ](/tpl/images/0400/0579/83f77.png)


