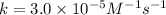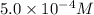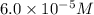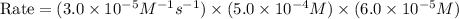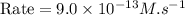Chemistry, 03.12.2019 01:31 PONBallfordM89
The reaction described by this equation
o3(g)+no(> o2(g)+no2(g)
has the following rate law at 310k.
rate of reaction=k[o3][no] k=3.0*10^6m^-1*s^-1
given that [o3]=5.0x10^-4m and no=6.0x10^-5m at t=0 calculate the rate of the reaction at t=0
what is the overall order of this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
The reaction described by this equation
o3(g)+no(> o2(g)+no2(g)
has th...
o3(g)+no(> o2(g)+no2(g)
has th...
Questions

Mathematics, 19.09.2020 01:01

Geography, 19.09.2020 01:01

History, 19.09.2020 01:01



Medicine, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01


Mathematics, 19.09.2020 01:01

Spanish, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01


Biology, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01



Geography, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01

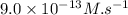
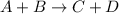
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0400/1843/10aeb.png)
![[A]](/tpl/images/0400/1843/6aa06.png) and
and ![[B]](/tpl/images/0400/1843/db909.png) = concentration of A and B reactant
= concentration of A and B reactant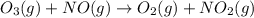
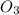 and
and 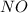 are the reactants.
are the reactants.![\text{Rate}=k[O_2][NO]](/tpl/images/0400/1843/1fdbe.png) ..........(1)
..........(1)