
Chemistry, 03.12.2019 02:31 madivassallo
What is the purpose of adding phenolphthalein to your erlenmeyer flask prior to starting a titration?
a. the phenolphthalein acts as a color changing indicator to signal the endpoint of the reaction.
b. the phenolphthalein will change color once we have added the correct amount of base to our acid.
c. the phenolphthalein acts as an acid in the neutralization reaction.
d. the phenolphthalein acts as a base in the neutralization reaction.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
What is the purpose of adding phenolphthalein to your erlenmeyer flask prior to starting a titration...
Questions



Health, 08.05.2021 21:40

Mathematics, 08.05.2021 21:50

Medicine, 08.05.2021 21:50


Computers and Technology, 08.05.2021 21:50

Mathematics, 08.05.2021 21:50

Mathematics, 08.05.2021 21:50


Mathematics, 08.05.2021 21:50


English, 08.05.2021 21:50

History, 08.05.2021 21:50


History, 08.05.2021 21:50

Arts, 08.05.2021 21:50

Advanced Placement (AP), 08.05.2021 21:50


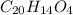 .
.


