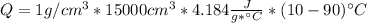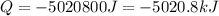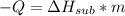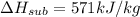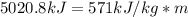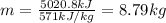Dry ice is solid carbon dioxide. instead of melting, solid carbon dioxide sublimes according to the following equation: co2(s)→co2(g). when dry ice is added to warm water, heat from the water causes the dry ice to sublime more quickly. the evaporating carbon dioxide produces a dense fog often used to create special effects. in a simple dry ice fog machine, dry ice is added to warm water in a styrofoam cooler. the dry ice produces fog until it evaporates away, or until the water gets too cold to sublime the dry ice quickly enough. suppose that a small styrofoam cooler holds 15.0 liters of water heated to 90 ∘c. calculate the mass of dry ice that should be added to the water so that the dry ice completely sublimes away when the water reaches 10 ∘c. assume no heat loss to the surroundings.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
Dry ice is solid carbon dioxide. instead of melting, solid carbon dioxide sublimes according to the...
Questions

Mathematics, 15.02.2022 21:00

Chemistry, 15.02.2022 21:00






Mathematics, 15.02.2022 21:00


Chemistry, 15.02.2022 21:00

Mathematics, 15.02.2022 21:00


Mathematics, 15.02.2022 21:00




Mathematics, 15.02.2022 21:00


Social Studies, 15.02.2022 21:00

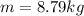
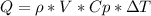
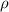 is the density
is the density